HIV: Progress and future challenges in treatment, prevention and cure
Natural Portfolio – Updated for October 2021
Advances in care over the last 30 years have helped transform HIV from a fatal disease into a chronic, manageable condition for many people. Innovations in treatment and access are at the core of this progress. The advent of antiretroviral therapy (ART), once-daily single tablet regimens (STRs) with three medications combined into one pill, and many subsequent improvements in STRs combining efficacy and long-term safety represent some of the most significant milestones that have helped bring radical change in the outlook for people living with HIV.
Progress in treatment has been transformative, but significant gaps in care still remain. HIV continues to be a major global public health issue. In the United States, the South accounts for seven of the top ten states and nine of the top ten metropolitan areas with the highest rates of new infections. This is pointed evidence that the epidemic is ongoing for some communities. Globally, it’s estimated that more than 36 million people are living with HIV with acute challenges faced by Sub-Saharan Africa and many low- and middle-income countries. With the goal of reducing the individual and societal burden of HIV, prevention of new infections is as important as treating those who are living with HIV.
At Gilead, we believe that anyone living with or at risk of HIV should benefit from the latest treatment innovations and have access to medicines. For almost 30 years, we have been at the forefront of the fight against HIV by leading advances in treatment, prevention and access. Our experience, dedication, and infrastructure are helping us move forward as we continue to focus on and make major investments in further expanding the options for HIV treatment and prevention. Equally as important, our close connections with the field, built over decades of collaborative research and development, enable us to help advance preclinical and clinical programs with the goal of achieving a functional cure for HIV.
Reducing Pill Burden with Single Tablet Regimens
In the early days of the HIV epidemic, high pill burden made successful adherence to treatment challenging. Selective non-adherence to a regimen increases the likelihood of developing resistance, which could limit future treatment options. STRs have revolutionized care by simplifying dosing and their impact has been profound and lasting. Today, clinical treatment guidelines from the US Department of Health and Human Services (DHHS) include certain STRs as recommended initial treatment regimen options for most people living with HIV. According to DHHS, factors that should guide the selection of a treatment regimen include virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance test results, comorbid conditions, access, and cost.
Despite the progress made in improving the efficacy and safety of HIV treatments, there are people with severely limited treatment options due to resistance to multiple ART classes. They rely on complex treatment regimens, sometimes involving injectable medications in combination with multiple pills several times a day. This type of complexity further increases the chance of treatment failure. Thus, we continue to strive to develop new agents that are active against resistant variants of HIV and target novel mechanisms of action, in order to provide simpler and more efficacious treatment options to all people living with HIV, irrespective of their prior treatment history. Our goal is to continue pursuing further ART innovations that enable STRs to reach beyond first and second line therapy options.
Researching Care with Long-Acting ART
Efforts to continue enhancing care will be critical to further curbing the HIV epidemic. Long-acting formulations with the potential to be dosed weekly, monthly, or even less frequently may help facilitate treatment adherence, a strong predictor of treatment success and health outcomes. To become suitable for long-acting administration, new agents need to fit criteria different from those applied to daily oral ART. Novel regimens must match the high efficacy and safety observed with currently approved HIV treatments and do so with administration that is ideally minimally painful and can be performed outside a doctor’s office.
Long-acting antiretrovirals have already been identified among integrase and reverse transcriptase inhibitors, but additional drug targets are needed to help realize the full potential of long-acting ART for a range of patients. Capsid, a multimeric shell present in infectious viral particles, is essential for virus replication as it plays crucial roles in both early and late stages of the viral life cycle. HIV capsid inhibitors represent a potential new class of antiretrovirals with high in vitro potency and very slow metabolic clearance leading to a prolonged half-life in in-vivo preclinical models. Importantly, treatment failure due to circulating or archived pre-existing resistance is unlikely in the setting of novel therapeutic targets. Optimized combinations of multiple genetically engineered broadly neutralizing anti-HIV antibodies might also represent a new class of agents with long-acting potential.
Pioneering Prevention Options
Prevention methods and practices are essential tools in the fight against HIV. The innovation of pre-exposure prophylaxis (PrEP), an approach that involves daily antiretroviral medication in combination with safer sex practices to reduce the chance of acquiring HIV in individuals at risk for HIV, has been widely accepted by community advocates and is included in World Health Organization (WHO) and US Centers for Disease Control (CDC) clinical guidelines as part of a comprehensive prevention strategy. Data are emerging to suggest the rate of new HIV infections can be dramatically reduced when PrEP and Treatment as Prevention (TasP) approaches are broadly adopted.
Since the introduction of PrEP as a prevention strategy, there have been ongoing initiatives across industry, government and community to help drive successful implementation, ranging from building awareness and improving persistence among communities disproportionately impacted by HIV to encouraging appropriate use through daily adherence. Despite an increase in PrEP uptake over the last five years among men who have sex with men, use among women has remained relatively low. The reasons are likely multifactorial; however, providing additional PrEP treatment options, such as long-acting agents, could have an impact on engaging women at risk for HIV. In this context, innovations in long-acting ART have the potential to be extended from treatment to prevention.
Continuing Innovation in Treatment and Prevention Access
Today’s access challenges will require continued evaluation and innovation. Diligent and responsive programs that regularly refine their approach to access scale-up will have the most success.
In the US, this requires industry leadership and close coordination with community partners as well as federal, state, and local policymakers. Early stage diagnosis and treatment initiation, connection to healthcare, and community support are key factors. Gilead’s FOCUS (Frontlines of Communities in the US) program was created in 2010 with the goal to develop and share screening, diagnosis and linkage to care best practices with hospitals, community health centers and community-based organizations in accordance with guidelines recommended by the CDC, US Preventative Services Task Force (USPSTF), and state and local public health departments. Through September 2018, FOCUS partners have conducted more than 5.1 million HIV tests from 261 participating organizations in 83 cities and counties.
Globally, the need is greatest in resource-limited countries with low HIV awareness and lack of education about treatment and prevention options. Since 2006, Gilead has entered into voluntary licensing agreements with generic manufacturers in low- and middle-income countries that grant them the rights to produce and sell high-quality, low-cost generic versions of Gilead medicines. Today, these agreements have expanded access in 116 countries and resulted in an 85 percent reduction in local prices due to manufacturer competition. We also partner with NGOs, governments, and academic institutions to raise awareness, address barriers to treatment access, reduce stigma and deliver frontline services. For example, in Africa, our work with the PEPFAR’s DREAMS initiative provides PrEP medications to young women who are most vulnerable to HIV.
Cure as the Ultimate Goal
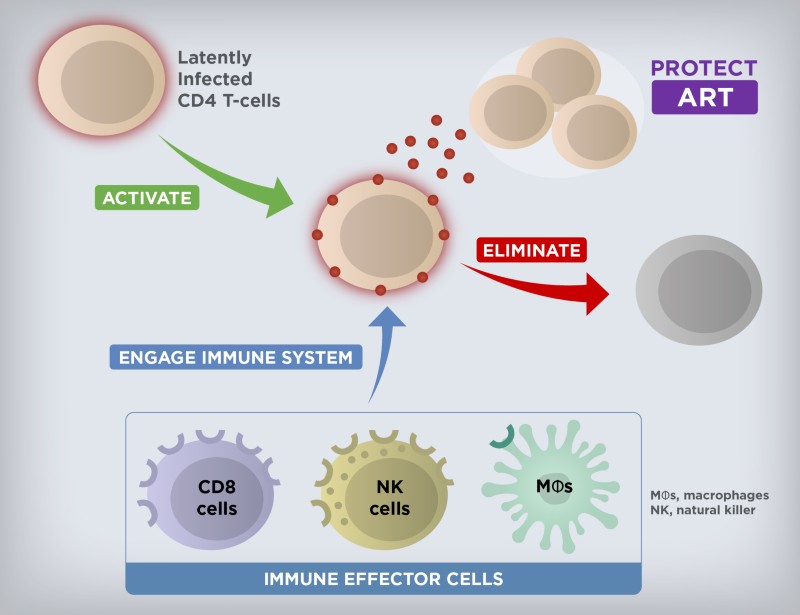
While still elusive, cure remains the ultimate long-term goal for Gilead’s HIV research and development efforts. It is well established that the latent HIV reservoir is the main obstacle in achieving cure. While the total size of the HIV reservoir varies among people living with HIV, the latently infected cells are rare (approximately 1 to 100 per million resting CD4+ T cells in the blood). The reservoir is also anatomically heterogeneous and persistent, due to the residence of latent HIV primarily in long-lived memory CD4+ T cells. The quiescent nature of these memory CD4+ T cells makes the reservoir essentially invisible to the immune system and not susceptible to ART. However, if ART is stopped, the virus quickly rebounds, leading to spreading HIV replication and expansion of the reservoir in the vast majority of individuals.
A multi-pronged approach will likely be needed to achieve the goal of curing HIV. The discovery and development of latency-reversing agents, immune modulators, and genetically engineered effector antibodies, as well as therapeutic vaccines with a potential for combination therapy to activate and eliminate the persistent reservoir are a current area of focus (Figure 1). Initial results from testing a combination of a TLR7 agonist with a broadly neutralizing antibody or a therapeutic vaccine in non-human primates infected with a chimeric simian-human immunodeficiency virus or simian immunodeficiency virus, respectively, have been a source of cautious optimism and helped advance several investigational agents into early-stage clinical studies. As we progress further with testing investigational curative regimens, our partnerships and collaborations are more important than ever in this complex effort.
Extraordinary progress has been made in HIV since the epidemic emerged 37 years ago, but significant challenges still remain ahead of us. Gilead is strongly committed to driving the next generation of treatment, prevention, and cure strategy innovations that will continue changing the trajectory of the HIV epidemic by transforming care and improving overall outcomes for all people living with or at risk of HIV.






