Alzheimer’s Nasal Vaccine, Protollin, in Clinical Trials
By Simon Spichak, MSc updated for January 2022
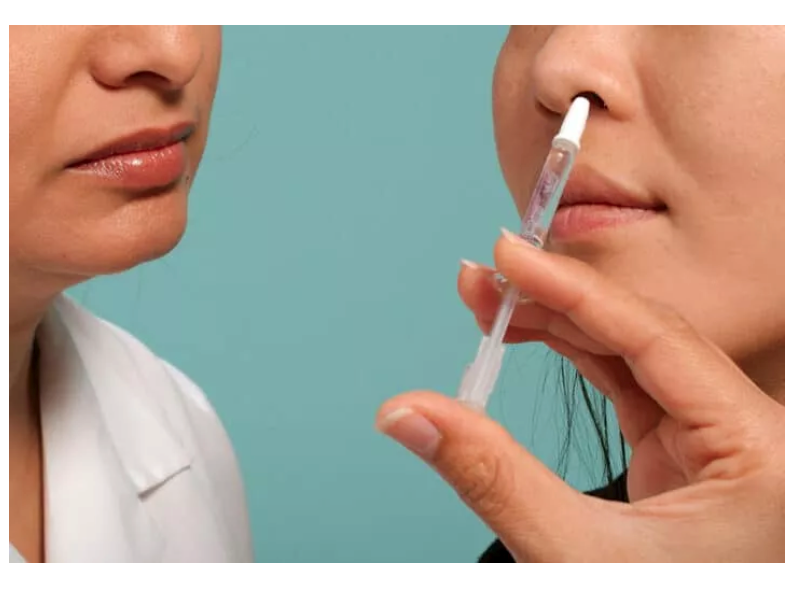
Protollin, a new vaccine for Alzheimer’s that is administered nasally, heads to human clinical trials.
Through the incredible power of our immune system, researchers are developing new drugs and treatments to tackle Alzheimer’s disease. Aduhelm, an anti-amyloid antibody-based drug was approved in June and other similar therapeutics, including gantenerumab, donanemab, and lecanemab, are currently in Phase 3 clinical trials. Vaccines provide another immune-based strategy to potentially modify and prevent Alzheimer’s.
There are at least nine vaccines in various other stages of clinical trials. Now, a new vaccine called Protollin — administered through a nasal spray — was recently approved to enter early human trials.
Some Alzheimer’s vaccines in the development pipeline now target specific misfolded proteins that are known Alzheimer’s biomarkers, such as tau or beta-amyloid. Protollin, however, doesn’t target these Alzheimer’s proteins.
Instead, it stimulates the innate immune system which contains cells called macrophages and microglia that eat away debris. For this reason, Dr. Tanuja Chitnis, physician, professor of neurology at the Brigham and Women’s Hospital, and principal investigator of the trial, explained, the approach could eventually prove useful for other diseases with misfolded proteins like Huntington’s or Parkinson’s disease.
“Only recently has the immune system been used to try to treat Alzheimer’s,” Chitnis told Being Patient. “I think this is a brand new phase [for developing Alzheimer’s treatments], we saw this change occur in cancer treatments 20 years ago.”
“We think that in Alzheimer’s, these cells are not doing their job sufficiently,” she continued, noting that “protollin is made up of several proteins, one is an outer membrane protein from a bacteria called Neisseria.” This excites the innate immune system, she said, helping it to eat away amyloid plaques that have formed in the brain.
Protollin’s Phase 1 clinical trial will assess the safety and tolerability of the vaccine as well as its effect on white blood cells. Participants are between 60 and 85 years of age, with early symptomatic Alzheimer’s and beta-amyloid plaque buildup in their brains according to PET brain imaging.
Will this Alzheimer’s vaccine require boosters? “In different mouse models, using the drug every few weeks can help to eliminate and reduce the beta-amyloid plaques,” Chitnis said. “It’s possible that this could be a drug that could be dosed once a month, or currently, we’re giving two doses over a two week period.”
Vaccines and other drug treatments could prove synergistic, she added, proving more effective when combined.
“There could be down the road room for two or more drugs to be used at the same time that are targeting different mechanisms in Alzheimer’s,” Chitnis explained.
Protollin or other vaccines could also be used to halt the process at the first signs of the disease. But according to Chitnis, there aren’t currently any biomarkers sensitive and specific to help diagnose the disease as early as age 40 or 50.
“There is the potential that this type of approach and maybe even this therapy could apply to other diseases with misfolded proteins,” Chitnis said.





